For
Lab
Manager

Your challenges as a laboratory manager
Let’s face it – the demands on your laboratory work are constantly growing. Increasing amounts of data, strict regulations and careful sample storage are increasingly challenging you and your staff. The processes and requirements for you as a laboratory manager are becoming more complex, the degree of difficulty in complying with guidelines and standards is increasing, as is the amount of instrument data that is being generated.
At the same time, your company demands that you increase the performance of the lab and turn your lab into a profit center.
On an ongoing basis, you need to initiate optimization measures. Above all, shortening the lab turnaround time is considered one of the major challenges. In addition to in-depth reporting and ever more comprehensive reports, the pressure to avoid errors, for example, is growing. This primarily concerns your certificates and test reports.
There is also an increasing need for higher data availability and integration in the laboratory. For example – everyone in the team and the departments involved would like to be able to access all relevant data sets from anywhere, and they would like less work through automated processes. The goals frequently mentioned by our customers here probably also coincide with yours: the reduction of administrative activities. The increase in efficiency – especially in resource planning, as well as the avoidance of bottleneck effects, mainly in the release process.
Many Lab Managers we know had previously used different software tools as isolated applications (e.g., Office applications, e.g., Excel & Access). They were used as test and measurement equipment, which were not directly compatible with each other, and they transferred and exchanged the data manually. This increased the risk of errors during input and transfer and cost valuable time.
At the same time, test data and reports must be prepared and forwarded to the various team in the company increasing pressure to be punctual, error-free and meaningful, while at the same time, meeting shorter deadlines requirements.
Moreover, you need to drive the change-management-process and modernization of the lab. In short, the solution to your increasing challenges in the laboratory could be a modern and highly integrated software platform that is networked with your entire quality management and all stakeholders in your company.
This is where we come in – providing you with the solutions to better manage your challenges.
Why
many customers choose the
QDA laboratory information
management software
(LIMS)
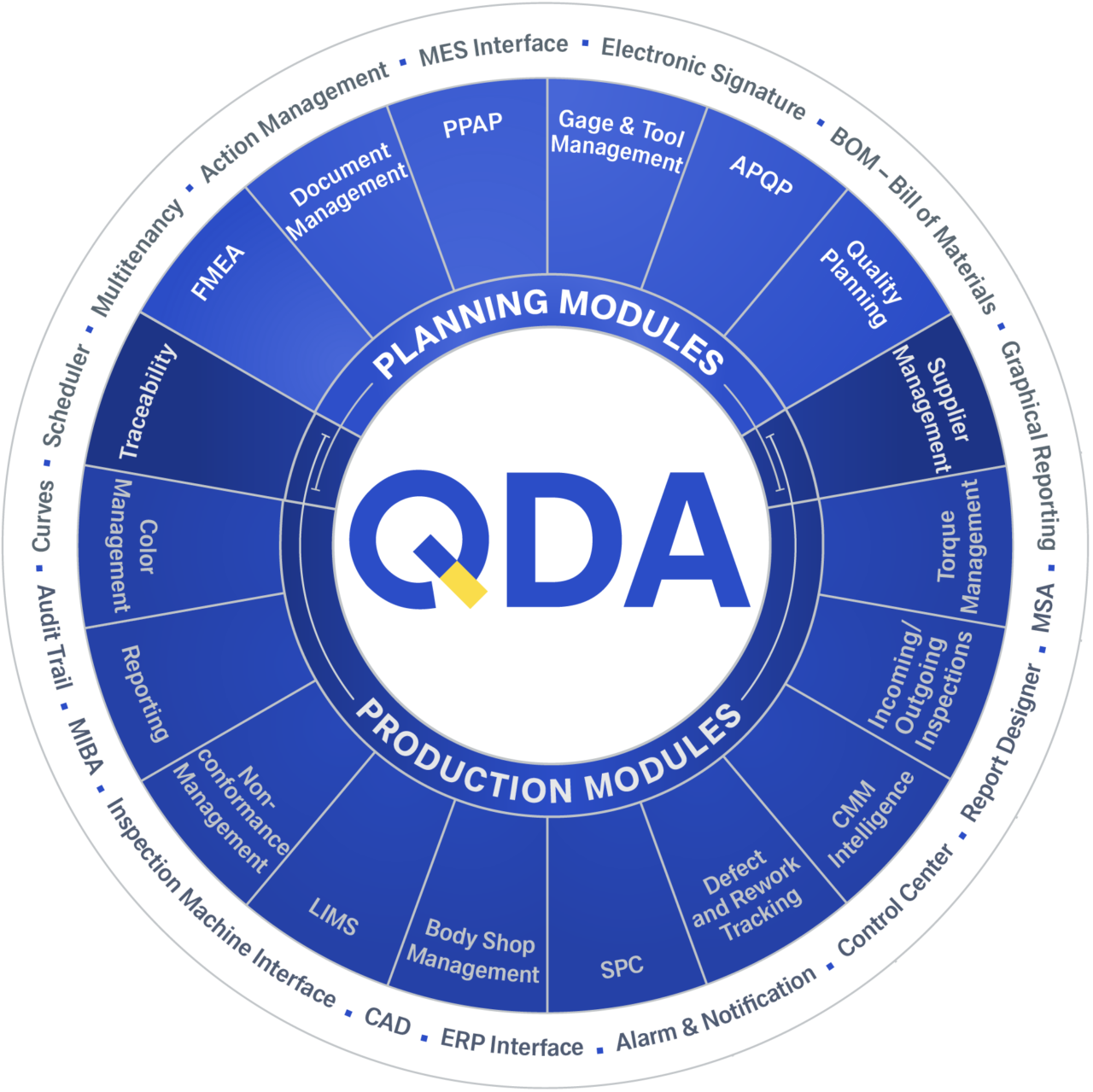
- Trusted references: Laboratory managers in global companies with plants across various countries have trusted QDA software for decades
- Increases efficiency and simplifies the collection of laboratory data
- Optimizes the order management of your quality control laboratory
- Speeds up your processes: You only need 1/4 of the time you used to spend on creating new test plans and creating orders with LIMS order management, creating certificates or lab reports
- More transparency
You can also benefit from the functions of QDA gauge and tool management

QDA gauge and tool management software improves cost control, traceability of measurements, tools and processes.

Electronic signature for laboratories

Die elektronische Signatur ermöglicht die elektronische Freigabe von Vorgängen für Prüfpläne und Verwendungsentscheidungen. Der Ersteller der Signatur wird identifiziert und die Integrität der elektronisch signierten Informationen werden wird nachgewiesen, was eine handschriftliche Unterschrift auf einem Papierdokument ersetzt. Die elektronische QDA-Signatur unterstützt die Systemkonformität mit FDA 21 CFR Part 11.

Learn more about industries in which help our customers be successful