Document Management System

What is document management in context of QM?
A standard compliant DMS controls the lifecycle of your documents in a 100% traceable manner. The result is an audit-proof information system in which a wide variety of users can access the current status of the documents which are relevant to them. User roles and approval processes can be adapted to the specific needs of a company: The process owner can define and track which group of people has access to which topics. At the same time, it is possible to check whether the documents have actually been read. In addition, qualification tests provide information on whether the content of the document has also been understood.
Disadvantages for production without DMS
Weak point number 1 are risks in the certification process: incomplete documentation is the most common reason for failing compliance audits. Regardless, document control in many places runs on collaboration software such as Sharepoint. QM-related workflows are not mapped in these programs. Instead, heterogeneous filing and process structures are created that become increasingly complex and confusing over time. This severely restricts the legally compliant handling of QM documents. At the same time, high process costs are incurred in handling. In addition, there is a constantly increasing risk that employees will work with outdated or contradictory documents and jeopardize the quality of their work.
The analysts’ view
Usage analyses make it clear that in many places it is disproportionately time-consuming to find or recover documents. For example, Gartner Research found that in many organizations, up to 40% of work time is spent retrieving information. Add to that four hours per month spent searching for documents and another six hours spent recreating lost documents. It is therefore not surprising when Gartner sees DMS as one of the most important fields of action for process optimization.
Higher process reliability and faster workflows
Our DMS software QDA directs your organization’s documents through standards-compliant workflows, creating uniform filing structures and ensuring easy, cost-reducing use. It standardizes, secures and automates the entire lifecycle of your documents. All status changes of a document are logged seamlessly in the audit trail. The support ranges from the creation of documents to their release and publication to usage analysis (qualification tests).
- QDA users find documents twice as fast as in conventional file structures.
- Templates for document creation reduce the workload of authors by one third.
- Delays in the release process are reduced by up to 20%.
- Integrated alarm and notification functions ensure that documents are processed on time and in full. Time savings are 20% and more.
Advantages of our integrated DMS software
- Integration with the other QDA modules: QDA DMS makes all quality management documents accessible. A single point of truth is created for the entire value chain.
- Role-based work: QDA DMS maps the needs of all user groups with suitable workflows.
- Traceability: Complete traceability. Even documents that have already been deleted can be retrieved.
- Version security: The version history of all documents is fully traceable.
- Integration at machine level: QDA DMS links all documents with specific links that web-enabled end devices can open online. This makes it possible, for example, to display test instructions on the operating displays of production systems.
- Standards compliance: QDA DMS can be used within the scope of the following standards and guidelines and supports the processes mentioned therein: ISO 9001:2008, ISO 14001, ISO/TS 16949, FDA 21 CFR Part 11 and VDA.
QM integration creates transparency for the entire value chain
Our DMS ensures that all documents created or maintained in the other QDA modules are entered into the document archive in compliance with standards. The archive serves as a central knowledge repository for the entire company. If, for example, a new FMEA or PPAP is created, these documents can be automatically entered into the DMS, where they are assigned a unique version number. The DMS organizes the distribution of documents according to roles. If release processes are required for this, corresponding workflows can be stored and executed automatically. A GMP-compliant signature function ensures that employees can authenticate themselves securely as part of integrated approval processes.

Functions of our DMS software
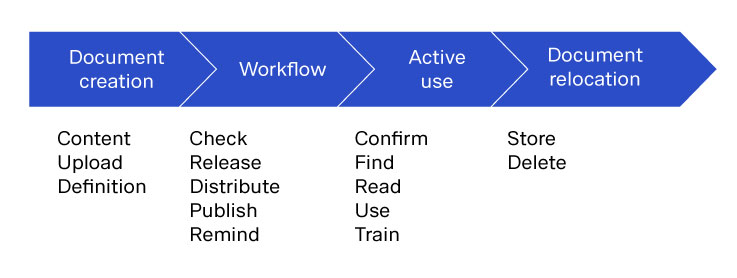
Life cycle of a document
Integration of DMS software – Freedom of choice in installation and use
Saas
All you need is a login: All inclusive – QDA and database optionally on Amazon Workspace, Azure, Oracle, Citrix, Terminal Server etc.
On Premise
We install QDA DMS in your IT environment.
Cost-optimized IT operation
Since QDA DMS can be used as a SaaS solution, you can significantly reduce the infrastructure and personnel costs of your IT organization. The more sites that access the DMS, the greater the savings.
Role-based access control
QDA DMS supports standard-compliant assignment of access rights. If users, roles and authorizations have already been created in other IT systems, automatic transfer is possible.
Maximum availability and data security
Thanks to its Kubernetes architecture, QDA DMS is 100% platform-independent. For customers who want maximum availability and data security, QDA recommends using a technology-leading cloud hyperscaler (e.g. Amazon, Azure, Google or Ionos). Additional security is provided by regular backups that QDA DMS creates on-site at the user’s location.
Data protection
QDA DMS complies with all the requirements of the European General Data Protection Regulation (GDPR).
Get in touch!
Do you have specific questions about our solutions? Get in touch!

Ludmila Lebedev
Director QDA SOLUTIONS