For
Quality-
managers

On this page you will learn which solutions are available for your quality management challenges and why our customers are so enthusiastic about our QMS software.
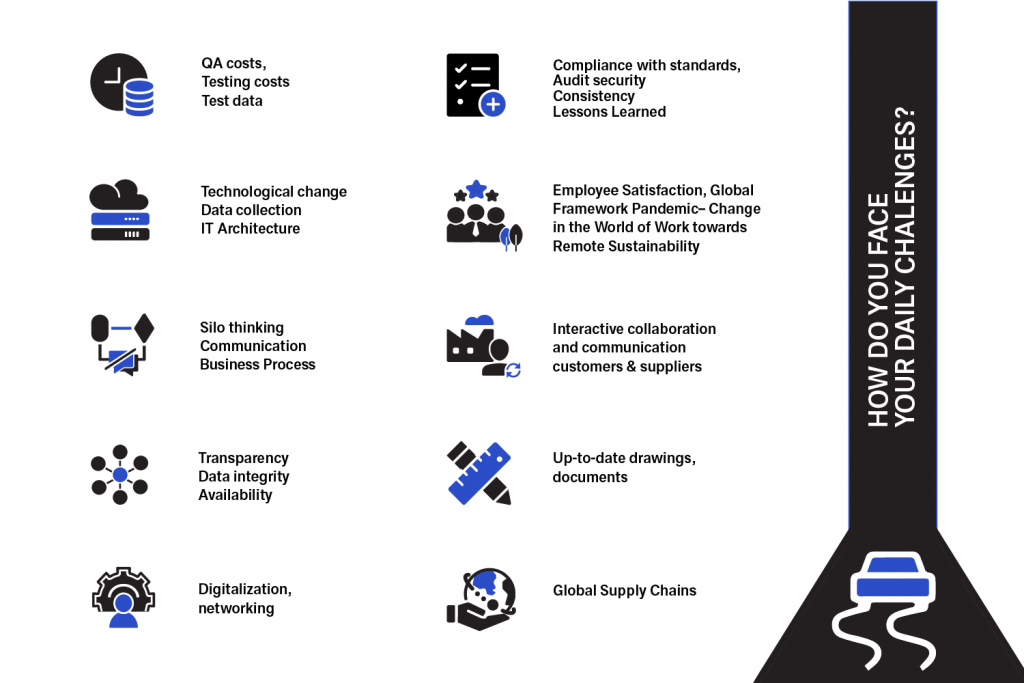
What are the challenges for quality managers?
In some companies, the tasks and challenges of a quality manager are similar to those of a quality assurance manager. But what are the concrete differences between a quality manager and a quality assurance manager?
Here is a possible definition: As a quality manager, you want to ensure that your company achieves its full potential. You would like to focus not only on the quality of products and services, but also on all other elements of the value chain.
The tasks and challenges of your colleague Quality Assurance Manager, on the other hand, include all preventive measures that make potentially negative influences on the quality of the end product visible and eliminates them. Otherwise, there would be a risk of producing defective parts, even if all processes in the company are running properly.
As a quality manager, you work closely with product-related quality control and process-oriented quality assurance. The common basis for this is a seamlessly integrated quality management system that ensures improved customer loyalty and satisfaction, as well as increased brand value for your company.
In your role as a quality manager, you bear an enormous amount of responsibility and are often caught between the chairs – in other words, you operate around tension between different company interests.
Many quality managers we work with on a daily basis tell us that they need the following to persist and drive business success:
- More authority and resources – primarily for modernization and the strong expansion of quality management (topic Industry 4.0 = Quality 4.0).
- A full integration of QM topics into the other management disciplines.
- Motivation and support from the CEO for a greater share in the economic success of the company.
- Waste of productivity due to “silo thinking” and too much bureaucracy
- Increasing complexity
Also, we often hear people tell them “Hello Quality Managers, you need to hit your Quality ROI!” but what exactly does that mean?
Do you also see it as a very big challenge to prove the return on investment and the business success achieved? If so, you are like most of the quality managers we already work with.
Understandably so, because one reason why it is so difficult to calculate your quality ROI is the increasing complexity of holistic quality management approaches. Things are not as simple today as they were back then. At that time, investments were compared with savings in inspection effort and cost reductions due to fewer complaints in order to prove economic success.
The background here: When considering the profitability of quality management, a distinction is usually made between “standard quality management requirements” and “quality management projects”.
The ROI can be calculated relatively well for the typical standard QM tasks. These mostly include these tasks:
- Increase in productivity
- Application of new technologies and optimized forms of organization.
- Use of statistical methods, spot checks, routines in the cooperation with the workers
- Use of IT & software
- Proof of the increase in productivity of quality management with predefined key figures.
The ROI resulting from special quality management tasks is much more difficult to calculate. These are mostly temporary projects with a quality management focus. They are often change or strategy projects or the introduction of new processes or instruments. Nevertheless, we have included a portion in the calculator.
For these projects, there should be concrete budgets and targets that are aligned with the business objectives.
As a quality manager, you exercise the role of advocate for quality in your company. So ideally, your management will reinforce you in this role! In many cases, however, we have heard that top management does not sufficiently recognize the strategic importance of your role. Not infrequently, problems with complaints were not addressed over the head of the quality manager or the causes of errors were relativized in order not to lose existing customer projects. Possibly this also concerns the use of the latest methods, such as predictive analytics? The latest software for this is often first introduced in other departments instead of being used in quality management from the very beginning. This also includes expanding investments in the democratization of technology and processes in quality management for integrated collaboration with all areas of the company.
The evolution of your quality management system with QDA
Act flexibly:
You can use our QMS software flexibly. This means, for example, that you do not have to purchase all available modules and apps right away – you can also start with just one module. In any case, you receive our QDA platform as a uniform basic system on which you can build.
More transparency:
Automated closed-loop quality processes give you greater visibility. Everything can be tracked end-to-end, from design to marketing.
High degree of integration:
The high degree of integration is achieved technically through the deep networking of data, teams, and systems. Our QMS software is a central platform for your data: With us, you can dissolve data silos in quality management. Everyone involved can access the data at any time from anywhere with the appropriate apps. Managing your quality processes is easier with quality workflows that are directly integrated with product development. Cross-functional teams can identify quality issues directly through closer collaboration. This allows you to resolve issues faster on future projects, or even prevent them altogether. The use of predictive quality data analytics helps here in particular.
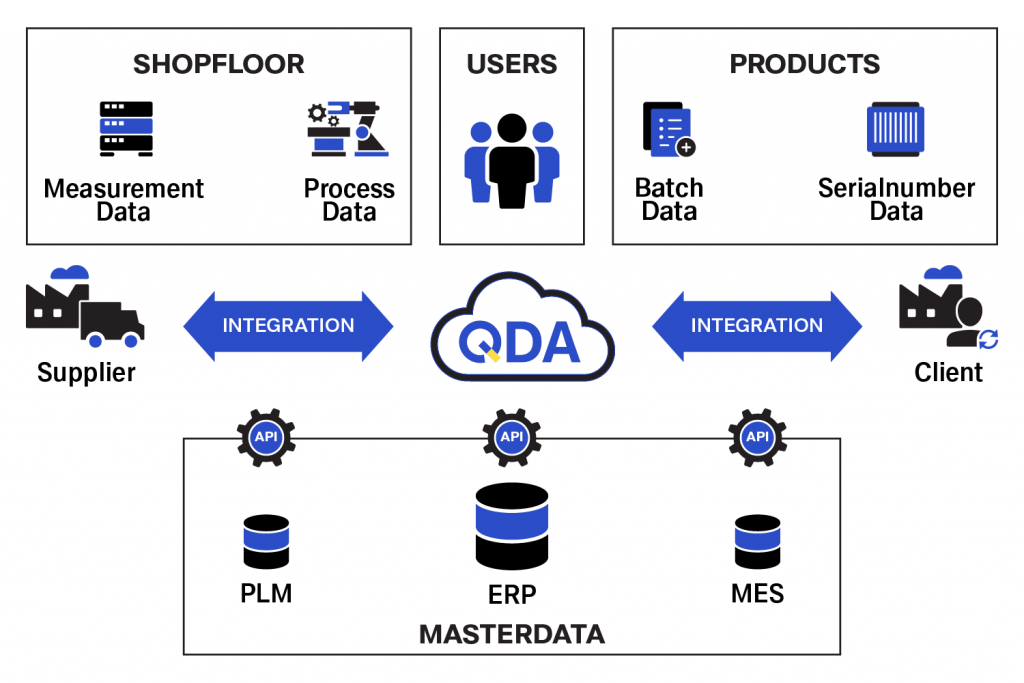
Upgrading processes for continuous improvement with QDA
You get even more in-depth insights into bottlenecks, delays, and trends of your quality management processes with QDA. Above all, the application of predictive quality data analytics facilitates the review and continuous improvement of quality processes in your company.
QDA accelerates the resolution of quality issues by matching and intelligently linking historical data (“lessons learned”) with measurement data in real time.
QDA inspires users in daily use -"easy and fun to use"
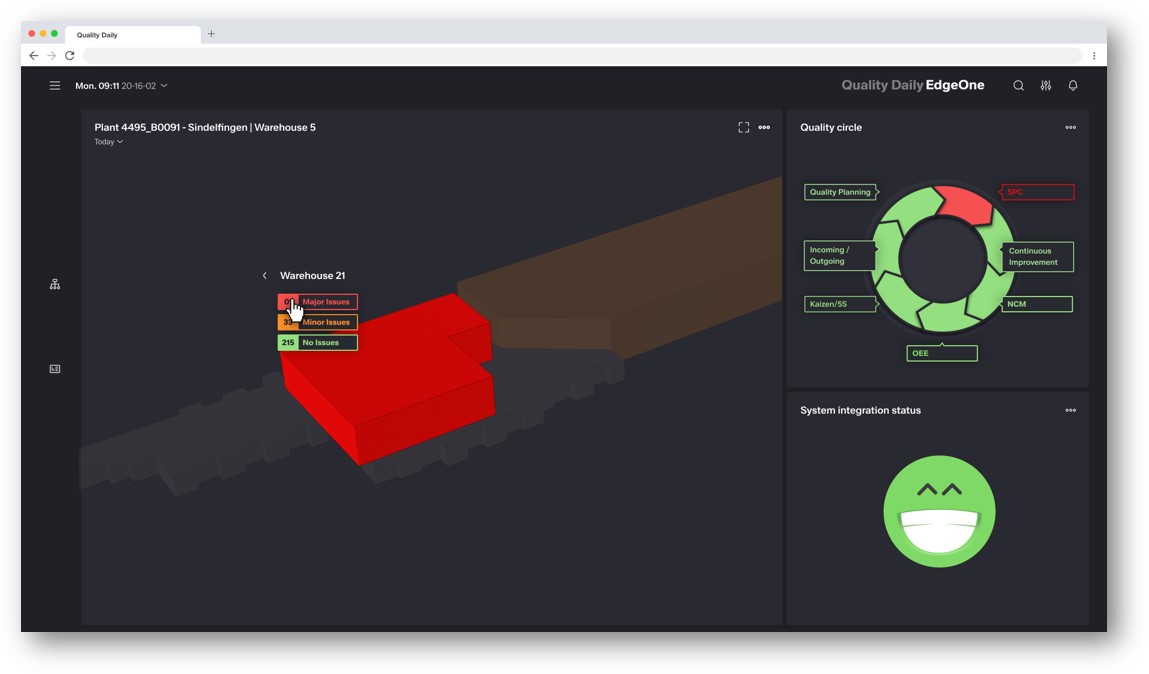

QDA is easy to use – “easy and fun to work with”. It has a modern interface with user and role based dashboard concept and extensive rights management.
E.g. Dark Mode possibilities – better for use in night shifts.


Why is QDA so widely used by global companies?
The central deployment allows you to start much faster than other systems. You can track the production quality of all company sites with a single solution.
Different plants can communicate with each other to improve production holistically and across sites. The data obtained forms the basis for a self-learning system.
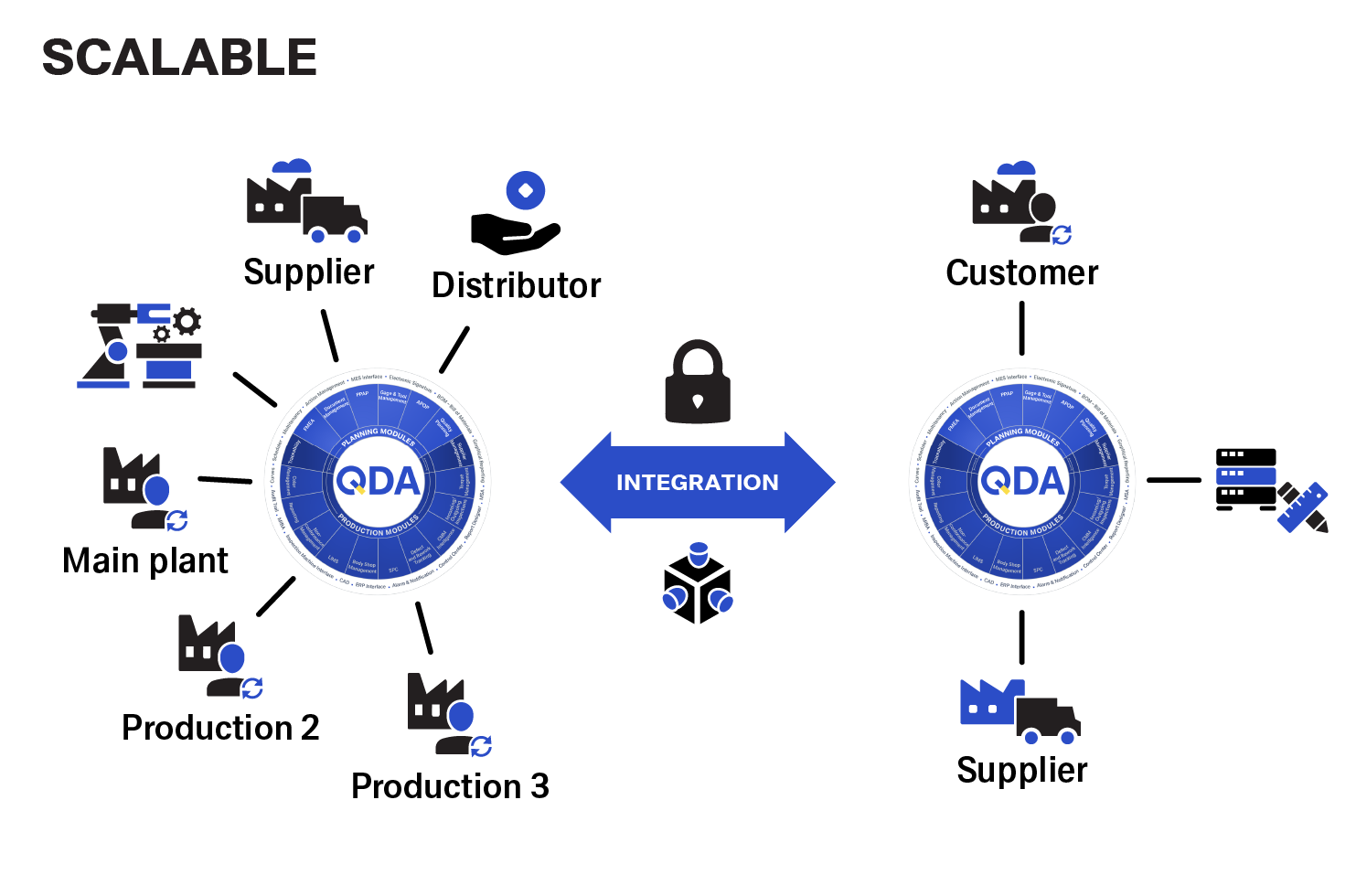
QDA is suitable for all your quality management tasks
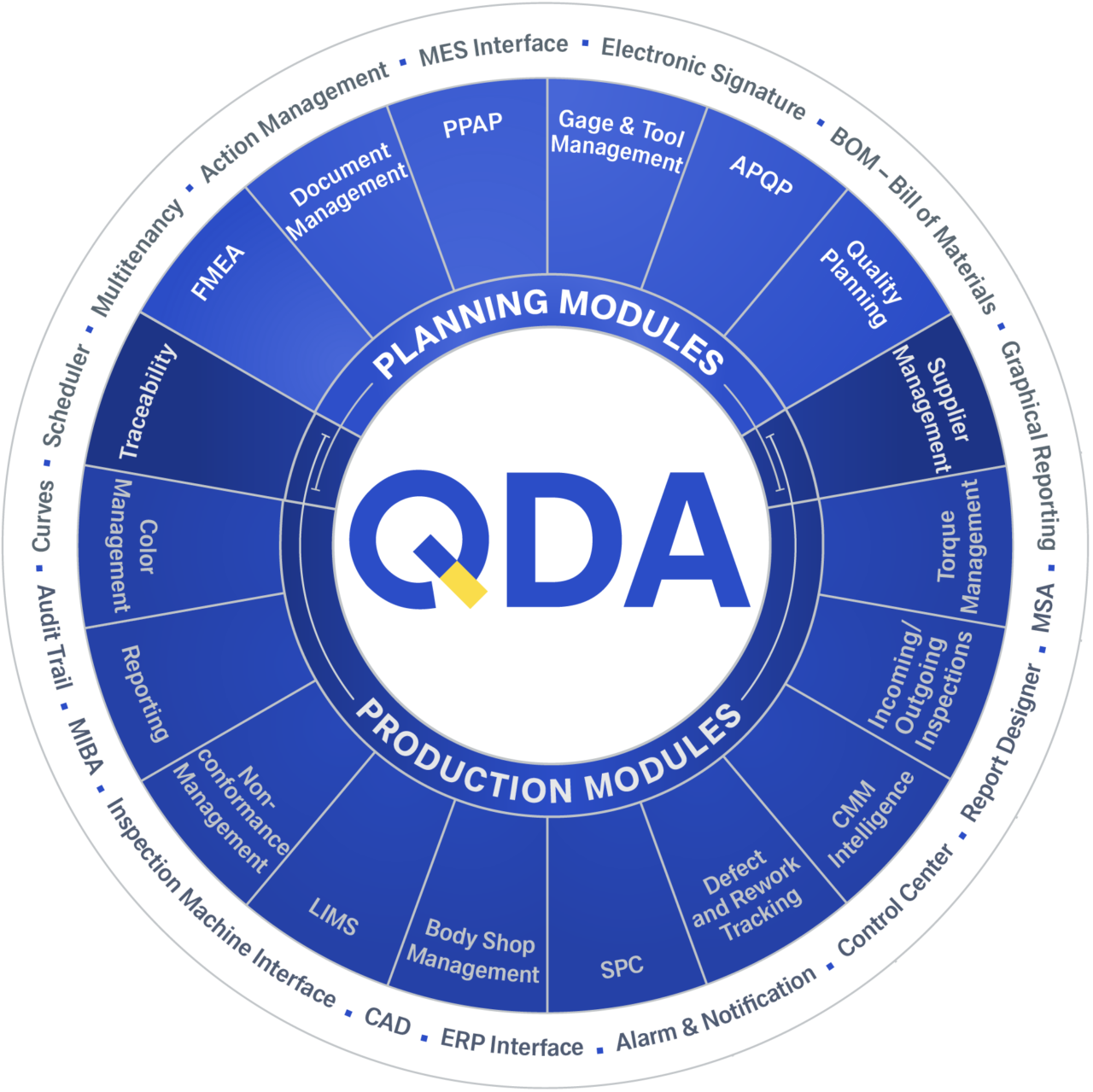
The QDA modules are divided into two groups. The planning and design area is covered, for example, by APQP, FMEA and PPAP. The results obtained from these flow into production modules such as SPC, LIMS or complaint management.
Our QDA solution is available on all mobile devices via app or web browser, data can be collected both online and offline. In addition, you can reduce the operational costs of QDA by using our QMS platform.

You want to see what QDA can do? Here you get an overview
The QDA SPC Software
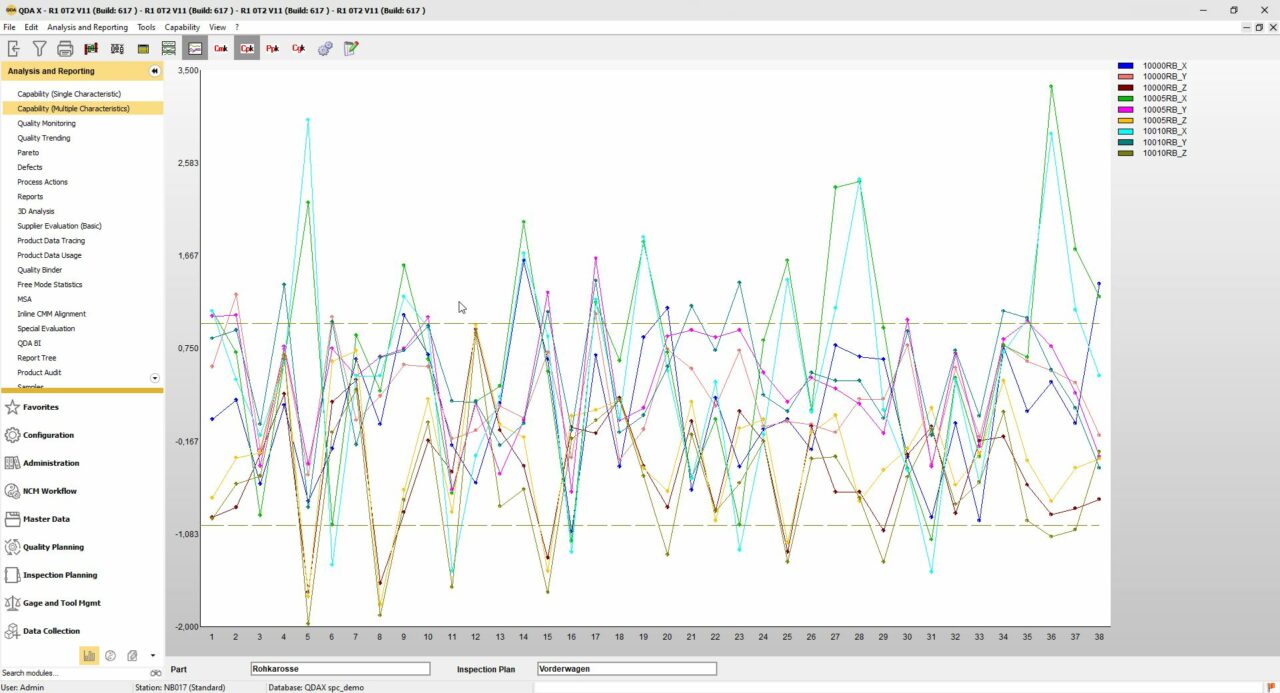

QDA is one of the most comprehensive data collection and SPC tools in the world. We know of no other solution that can collect and act on quality data better than QDA. Major tasks such as machine capability inspection (MFU), process capability inspection (PFU) and online process monitoring by quality control charts (QRC) are performed by our software to the complete satisfaction of our customers. It can automatically collect data from more than 500 different types of measuring devices and includes up to 100 different fields and traceability functions. QDA SPC has a very comprehensive statistical engine. The following elements in QDA are superior: reporting, free mode statistics, total statistics, import of data from gauges or systems and other strong features. As with all software modules, there is an optional selection of powerful tools.


For better quality with your Lab: The LIMS software from QDA
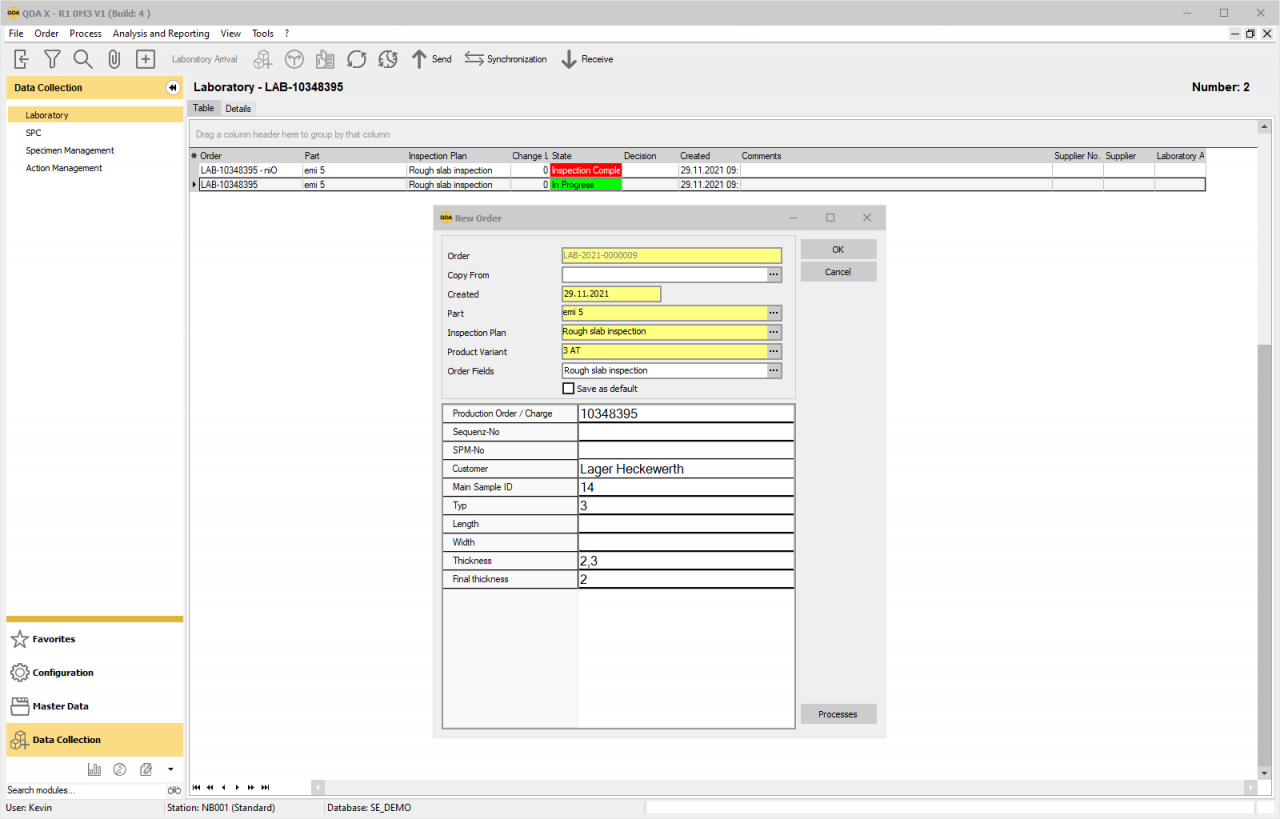

The demands on laboratory work are constantly increasing. Growing data volumes, strict regulations and careful storage of samples are increasingly challenging you and your staff.
If you use our LIMS software, your laboratory staff will achieve great time savings with the integrated resource management. Mainly through the integration of the QDA modules, the automated device connection, the creation of new test plans, the order creation and the creation of certificates or lab reports (up to 75% time saving). In addition, there is a high level of compliance with standards. There are also optional QDA tools here – one of them is called Audit-Trail, which is important for many of our customers and is used to control and record changes made in processes.


For Quality Managers: Our Complaint Management Software


With our QDA complaint management software, you quickly and securely identify all details of the problems that have occurred and the resulting complaints. With automated workflow definitions, you only need a few employees to achieve correct results. With QDA complaint management software, flex and ERP integrations are also possible and thanks to the integrated BI reports, you can analyze your business processes in detail. Moreover, QDA also integrates ISO standards and FDA mandates, combines CIP and CAPA, and consolidates and analyzes results. Based on an intuitive and targeted report, you decide on further steps with the goal of reducing your costs better than ever before.


The QDA Supplier Management Software for Quality Managers

With our supplier management software you improve, control and evaluate the relationships with your suppliers. QDA supplier management software complies with VDA and ISO 9001 guidelines and APQP, PPAP and PPR standards, giving you greater control and more sustainable improvements for managing your suppliers and their products. The supplier management software, in combination with our complaint management software, enables an integrated automated process that includes traceability to suppliers. In addition, high values in time savings, transparency and control characterize it.


QDA's Measurement Data Management for Quality Managers
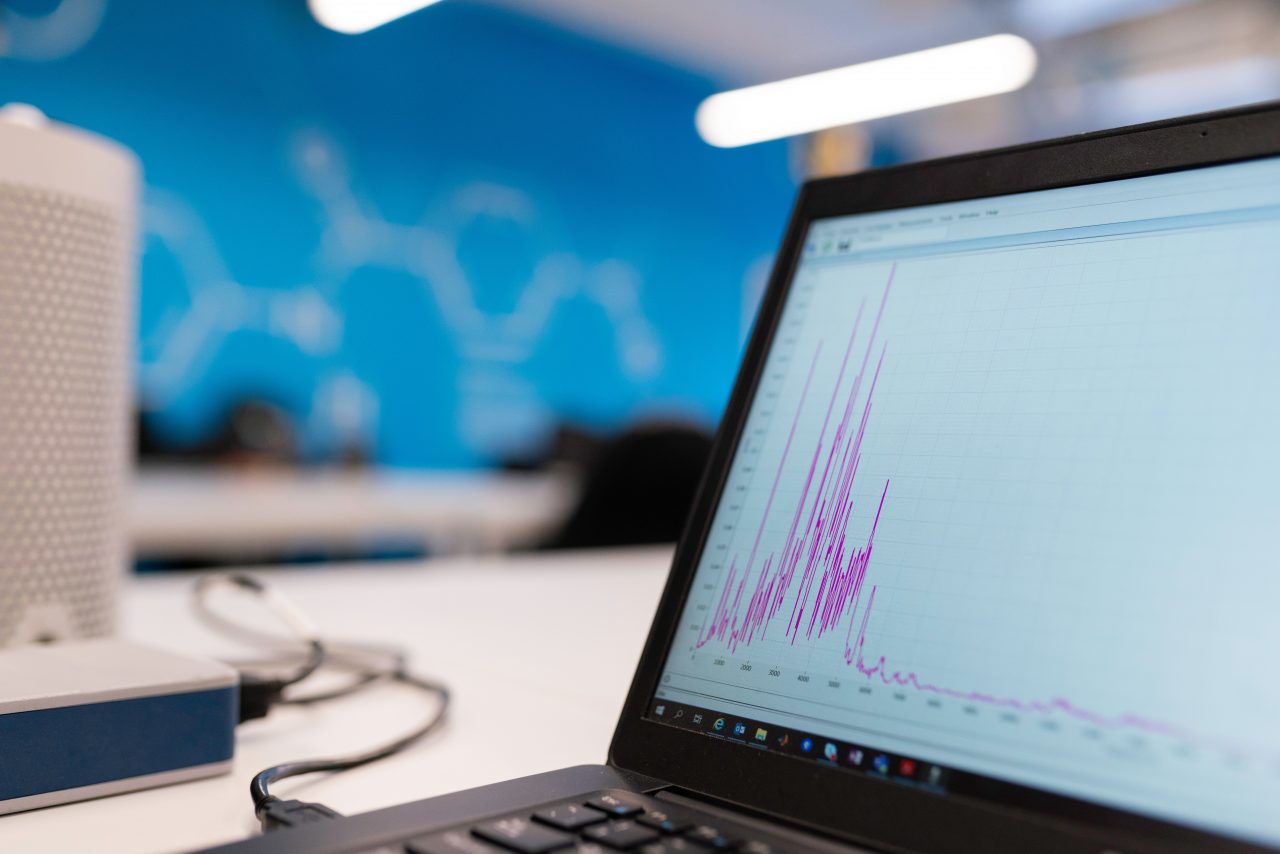

You would like to collect your measurement data centrally, visualize it clearly and control tolerances, measurement data and analysis sessions in real time? Our measurement data management software is well suited for this. Read more about the features and benefits below. Visualization is the biggest advantage of an MDM. It allows you to manage all your data in a single software system, detects errors faster and makes remedial actions taken more effective. A wide variety of tactile and/or non-contact coordinate measuring machines are used – online or offline. As an option to our measurement data management software, there is also a choice of powerful tools.


The Traceability Software for Quality Managers


Our traceability software supports you in proving when, where and how your products were manufactured or installed. This is how you ensure product liability and compliance with standards. QDA traceability software uses your product and process data to guide your business decisions. It’s easy to create analyses, and the integrated action management reduces errors, rework and warranty claims.


The QDA Calibration Management Software for Quality Managers
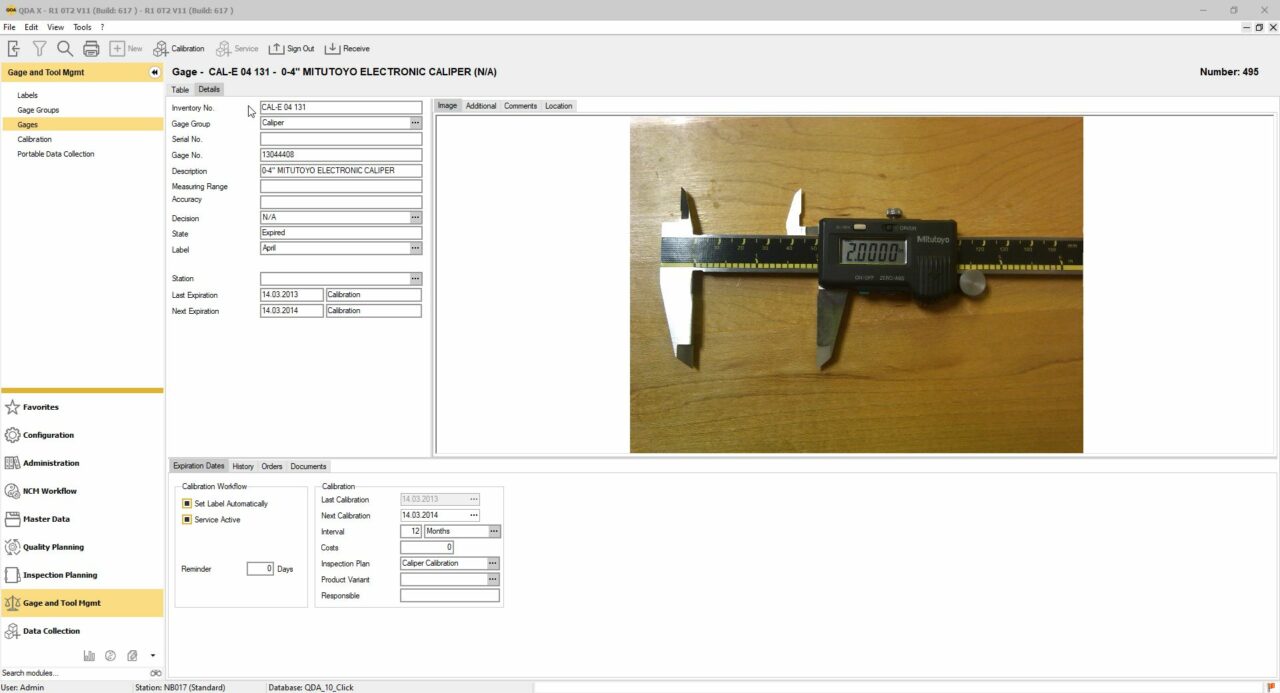

As with all QDA software modules, you’ll see tremendous time savings with our Calibration and Gauge Management Software. Whether it’s creating internal calibrations, sending out calibration lists, or traceability, it will always be significantly faster for you and your team than ever before. Much of this is also due to the deep QMS software integration with your other systems in your software landscape, e.g. MES / ERP.


The QDA APQP-Software for Quality Managers
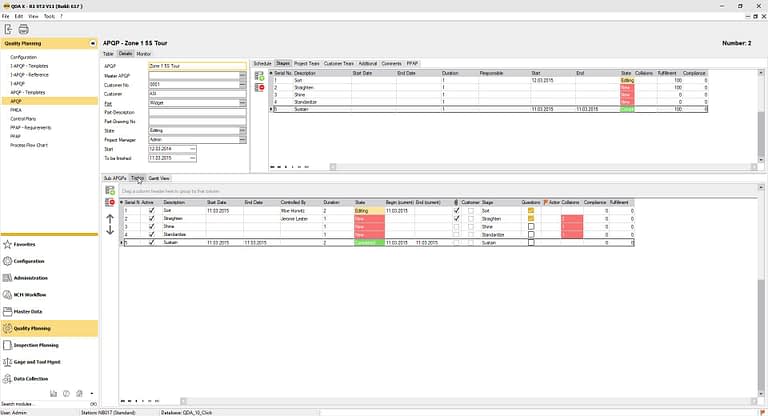

The software module integrates all APQP requirements and supports process-based quality management systems and continuous improvement. Other modules, such as design and system FMEA, PPAP and MSA can be linked to the quality planning module. You achieve enormous time savings, including in the creation of APQP reports with the integrated report designs and templates, as well as in the creation of inspection plans from control plans. At the same time, you reduce overall costs due to the high level of integration.


Learn more about industries in which help our customers be successful