About QDA SOLUTIONS

Excellence in Quality
QDA SOLUTIONS is one of the leading providers of quality management software (QMS). Headquartered in the beautiful and historic city of Lübeck, Germany, we have been developing software for over 30 years. QDA SOLUTIONS’s customer base includes world-renowned customers from a wide range of industries – as well as regional, small and medium-sized companies.
Our ambition
Fast results such as ensuring quality, reducing costs, optimizing processes – that is the promise to our customers. To achieve these goals, we always work closely with our customers. We are quality experts and our software solutions fully cover the classic disciplines of QMS. In addition, we want to help shape the quality management of tomorrow and pave the way for this future for our customers. The democratization of quality management in the ecosystem from customers to departments to quality management departments and suppliers is our focus. In doing so, we use machine learning technologies to gain extended insight from data of the entire ecosystem.
For a climate-neutral future

Climate change is one of the greatest challenges of our time. Companies should set a good example here. Sustainable business practices are becoming a social obligation.
We have recognized our responsibility and have continued to focus on the issue of sustainability in our company for a climate-neutral future. We not only use energy-saving equipment, such as laptops instead of PCs, and avoid other major power guzzlers, but have also switched to 100% green electricity.


The QDA SOLUTIONS History

1987
The company was founded in 1987, at that time under the name ddw Computersysteme. In the following years, outstanding successes were celebrated and a fully integrated QMS system was created.
2008
In 2008, ddw Computersysteme was taken over by ASI DATAMYTE and has thus in particular pushed ahead with internationalization. Our worldwide subsidiaries and partner companies have since enabled a roll-out on a global scale.
2017
After successful years of expansion, in 2017 our current parent Germanedge took over the QMS solution and team from ASI DATAMYTE. Since then, we have been operating as QDA SOLUTIONS and implementing the shared vision of Germanedge.
2020
QDA SOLUTIONS Nordics ApS is founded in Ringkøbing, Denmark.

The most important facts at a glance
Management Team
Since the end of 2020, Andreas F. Lüth has been the managing director of QDA SOLUTIONS. The extended management team consists of long-time QDA SOLUTIONS employees as well as industry experts: Randy Eichberg (Head of Customer Development), Claus-Dieter Jensen (Head of Product Development and Management), Nina Kopf (Head of Operations), Ludmila Lebedev (Head of Sales), Andrea Müller-Staffelstein (Head of Solution Engineering) und Paul Oxfeldt (Head of Region Europe/ Asia).
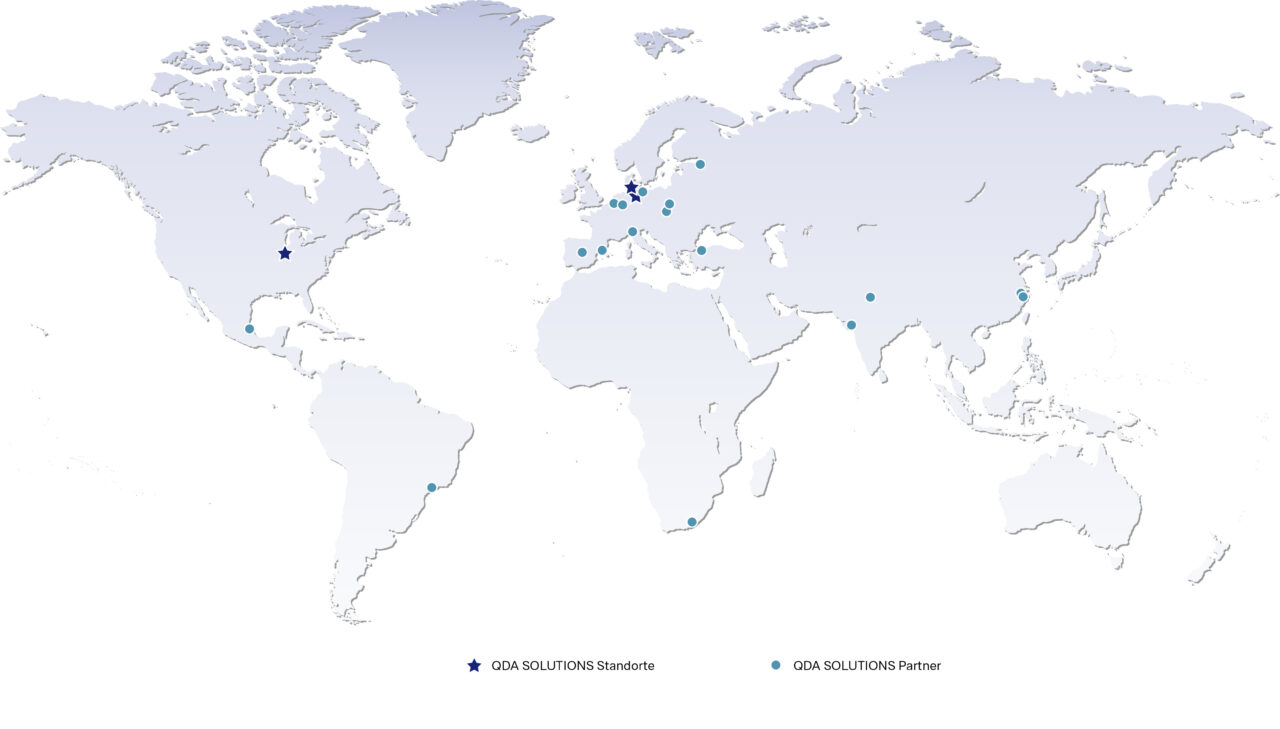
Our locations

Our corporate headquarter is located in Lübeck. Our product management, marketing, consulting, support, sales, finance, IT and HR teams work here. In addition, we are spread across the globe, for example in Ringkøbing (Denmark) and Bloomington (USA). There, we mainly work in customer-facing teams such as sales, consulting and support. Further locations can be found in the graphic.